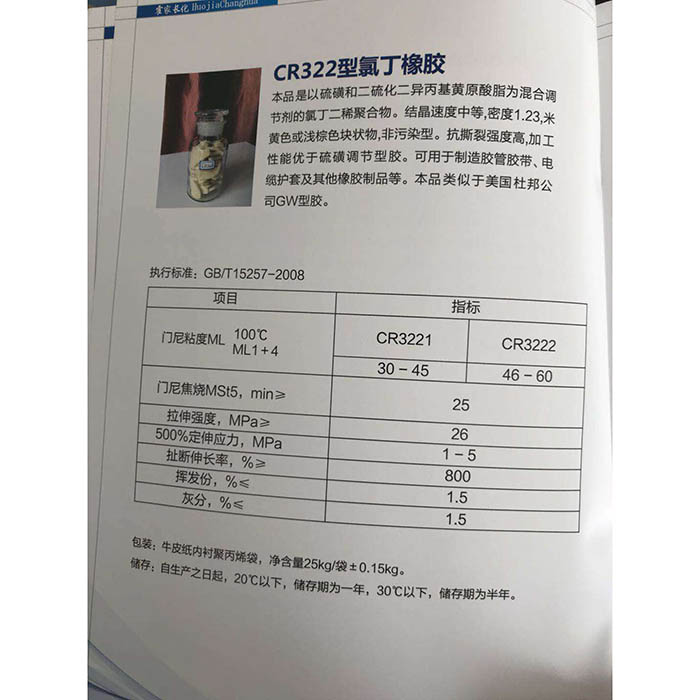
Sodium hydroxide
Sodium hydroxide, wanda tsarin sinadaran NaOH, wanda aka fi sani da caustic soda, caustic soda da caustic soda. Lokacin da aka narkar da shi, yana fitar da warin ammonia. Yana da karfi causticAlkali, wanda gabaɗaya a cikin flake ko granular form. Yana da sauƙin narkewa a cikin ruwa (lokacin narkar da cikin ruwa, yana ba da zafi) kuma yana samar da maganin alkaline. Bugu da ƙari, yana da lalacewa kuma yana iya ɗaukar tururin ruwa (deliquescence) da carbon dioxide (lalacewa) a cikin iska. NaOH daya ne daga cikin sinadarai da ake bukata a dakunan gwaje-gwajen sinadarai, kuma yana daya daga cikin sinadarai na gama-gari. Samfurin tsantsar ba shi da launi kuma crystal m. Yawaita 2.130 g/cm. Matsayin narkewa 318.4 ℃. Matsakaicin zafin jiki shine 1390 ℃. Kayayyakin masana'antu sun ƙunshi ƙaramin adadin sodium chloride da sodium carbonate, waɗanda fararen fata ne kuma lu'ulu'u ne. Akwai toshewa, ƙwanƙwasa, granular da siffar sanda. Nau'in adadi 40.01
Sodium hydroxideza a iya amfani da shi azaman wakili mai tsaftacewa na alkaline a cikin maganin ruwa, wanda aka narkar da shi a cikin ethanol da glycerol; Insoluble a cikin propanol da ether. Hakanan yana lalata carbon da sodium a matsanancin zafin jiki. Halin rashin daidaituwa tare da halogen kamar chlorine, bromine da aidin. Tsabtace da acid don samar da gishiri da ruwa.
Kaddarorin jiki na nadawa
Sodium hydroxide wani farin kristal ne mai tsauri. Maganin sa na ruwa yana da ɗanɗano astringent da jin daɗin satiny.
Nadewa deliquescence Yana da lalacewa a cikin iska.
Nadewa sha ruwa
M alkali ne sosai hygroscopic. Lokacin da aka fallasa shi, yana shayar da kwayoyin ruwa a cikin iska, kuma a karshe ya narke gaba daya zuwa mafita, amma ruwa sodium hydroxide ba shi da hygroscopicity.
Nadawa mai narkewa
Nadawa alkalinity
Sodium hydroxide zai rabu gaba ɗaya zuwa ions sodium da ions hydroxide lokacin da aka narkar da shi cikin ruwa, don haka yana da cikakken alkali.
Yana iya aiwatar da halayen tsaka-tsakin acid-base tare da kowane protonic acid (wanda kuma ya kasance na amsawar ruɓewa sau biyu):
NaOH + HCl = NaCl + H₂O
2NaOH + H₂SO₄=Na₂SO₄+2H₂O
NaOH + HNO₃=NaNO₃+H₂O
Hakazalika, maganinta na iya samun amsawar ruɓewa sau biyu tare da maganin gishiri:
NaOH + NH₄Cl = NaCl +NH₃·H₂O
2NaOH + CuSO₄= Cu(OH)₂↓+ Na₂SO₄
2NaOH+MgCl₂= 2NaCl+Mg(OH)₂↓
Nadawa saponification dauki
A yawancin halayen kwayoyin halitta, sodium hydroxide shima yana taka rawa iri ɗaya a matsayin mai haɓakawa, wanda mafi yawan wakilci shine saponification:
RCOOR' + NaOH = RCOONa + R'OH
Rushe wasu
Dalilin da yasa sodium hydroxide yana raguwa cikin sauƙi zuwa sodium carbonate (Na₂CO₃) a cikin iska saboda iska tana dauke da carbon dioxide (co):
2NaOH + CO₂ = Na₂CO₃ + H₂O
Idan ana ci gaba da gabatar da carbon dioxide da ya wuce kima, za a samar da sodium bicarbonate (NaHCO₃), wanda aka fi sani da baking soda, kuma ma'aunin amsawa shine kamar haka:
Na₂CO₃ + CO₂ + H₂O = 2NaHCO₃
Hakazalika, sodium hydroxide na iya amsawa da acidic oxides kamar silicon dioxide (SiO₂) da sulfur dioxide (SO):
2NaOH + SiO₂ = Na₂SiO₃ + H₂O
2 NaOH+SO (trace) = Na₂SO₃+H₂O
NaOH+SO₂ (wuce kima) = NaHSO₃ (wanda aka samar da NASO da ruwa suna amsawa da SO mai yawa don samar da nahSO)